If the FDA authorizes and ACIP recommends a booster dose people who were first to receive a COVID-19 vaccination when they became available in early 2021 eg those who are most at risk are likely to be the first people eligible for a booster. We have developed a plan to begin offering these booster shots this fall subject to FDA conducting an independent evaluation and.

Fda Vaccine Advisory Committee Rejects Biden S Booster Shot Plan For General Population Health News Us News
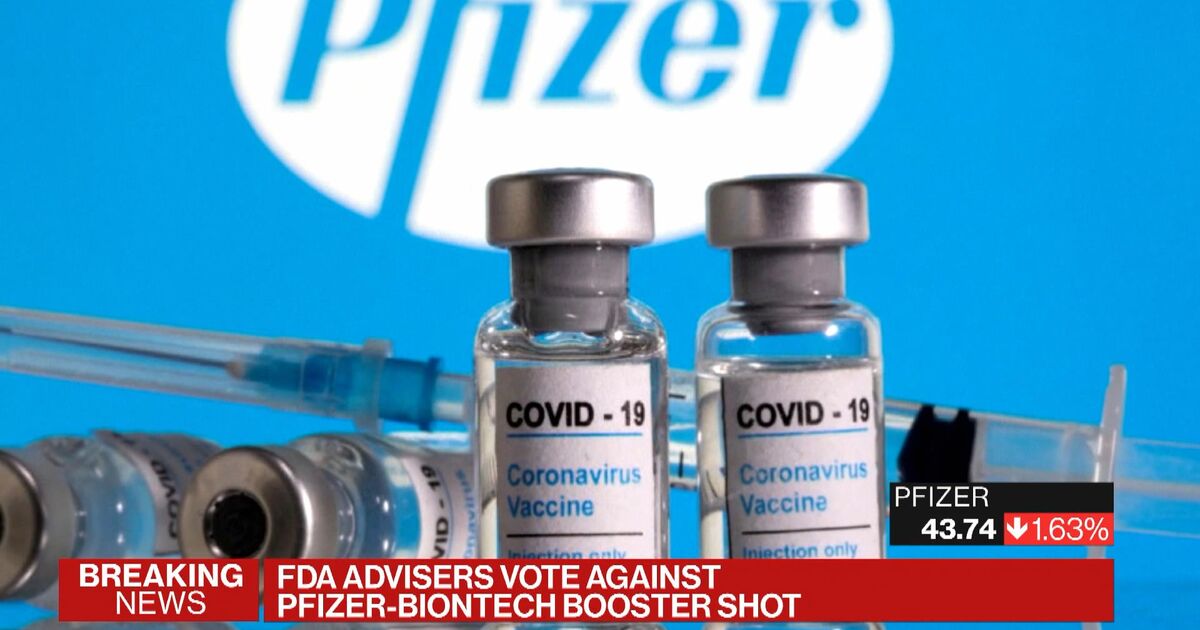
FDA announced a virtual VRBPAC meeting to discuss the Pfizer-BioNTech supplemental application for a third booster dose of Comirnaty COVID-19 Vaccine.


Fda Pushes For Moderna Booster Shot Data In Weighing Dose Bloomberg
Moderna Begins Submission Process For Fda Approval Of Its Covid Booster Shot Barron S

Moderna Asks Fda To Authorize A Booster Shot Of Its Covid 19 Vaccine Coronavirus Updates Npr

Fda Authorizes Covid Booster Shots For Certain Populations Medpage Today

Moderna Submits Preliminary Data To Fda For Covid 19 Booster Shot

Pfizer And Biontech Submit Early Data To Fda For Booster Covid 19 Shot







Post a Comment
Post a Comment